
Custom Antibody Production
Over the past 30 years, Scantibodies has developed an efficient, cost effective system of manufacturing monoclonal antibodies on a contract basis.
There are two types of custom monoclonal antibody production, in vivo (in mice), and in vitro (using cell culture). For cost, speed and scale up reasons, in vivo has been the most popular method.
Custom in vivo monoclonal antibody production is accomplished using a wide variety of cell lines. At Scantibodies, BALB/c mice are maintained in a breeding colony with a population up to 225,000 mice. Other strains of mice (Swiss Webster, CAF-1, ICR, etc.) are also available for antibody production. Custom in vitro monoclonal antibody production is performed in roller bottles.
There are three types of price plans available for custom MAb (Monoclonal Antibody) production. The price can be based on any one of the following:
Price Plans
By purchasing the MAb on the basis of purified, functional antibody, risk to the customer is eliminated. When a price quote is based on forms other than purified MAb (i.e., number of mice or roller bottles, volume of ascites, etc.), the final MAb price is indeterminate. Limits in hybridoma antibody production and losses incurred during the purification process can account for dramatically increased cost.
Custom MAb Production is a Multistep Process
• The client ships an existing hybridoma cell line to Scantibodies (SLI) utilizing SLI shipping procedures. Careful shipment ensures the safety of the cell line.
• SLI offers cell line optimization for maximum MAb production.
• Cell line drift, one of the greatest dangers encountered by a cell line, can be prevented with close monitoring and cell line certification.
• Custom MAb production is offered in ascites and, as an alternate method to in vivo production, SLI can propagate a large number of antibody producing cells in vitro using roller bottles.
• A customized, multistep purification process is performed to meet MAb specifications.
• Shipments of MAbs are accompanied by a detailed certificate of analysis. All procedures are discussed in detail in sections indicated on the flow chart.
Monoclonal Antibody Production
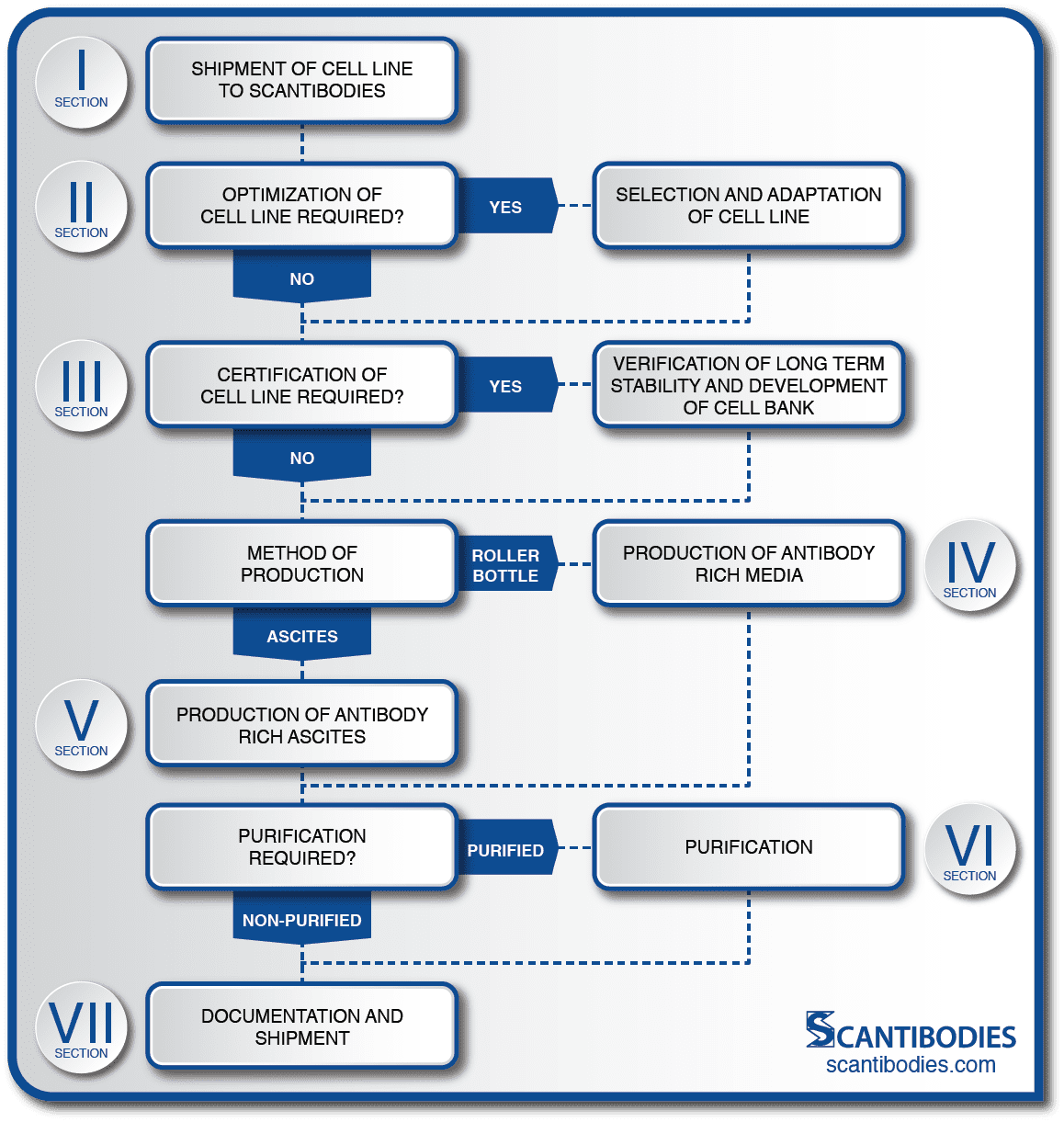
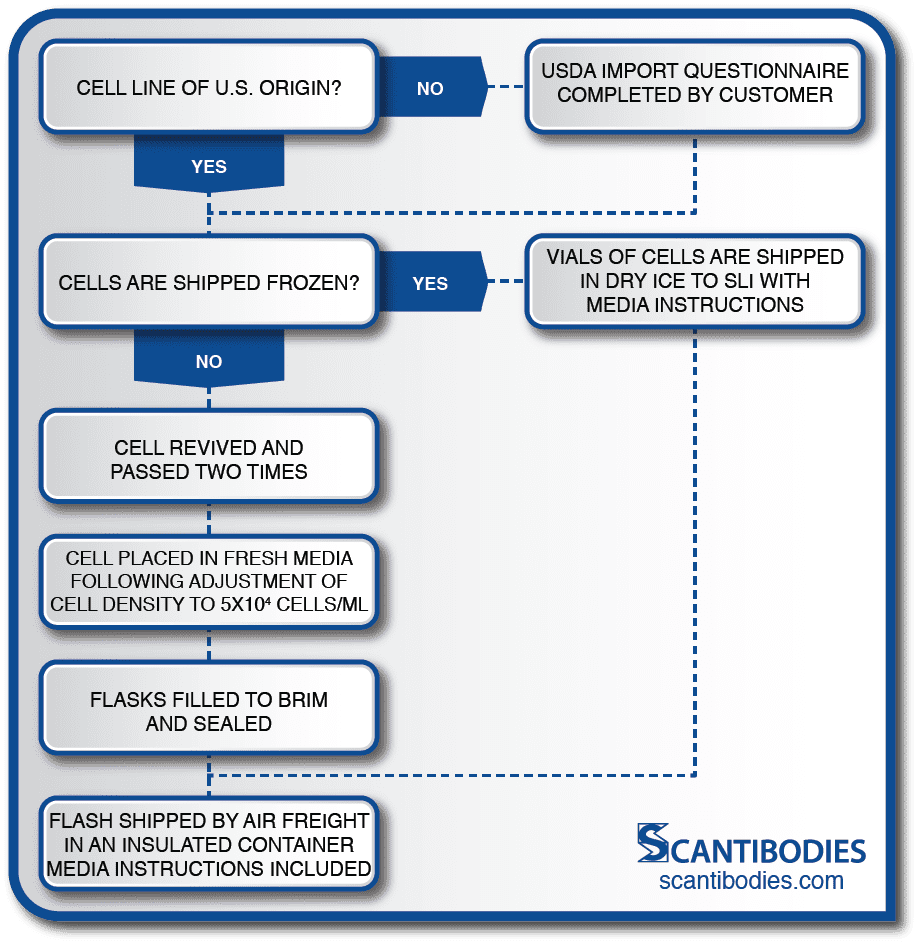
Section I: Shipment of cell line to Scantibodies
SLI Facilitates All Shipping Procedures to Ensure the Safety of the Cell Line.
For shipments from outside the U.S.A., SLI provides a questionnaire to the customer so that SLI may receive the shipment of cells.
If cells are to be shipped as live culture, the customer must revive the cells and check for viability. The cells are then passed two times and placed in fresh media following cell density adjustment to 5×104 cells/mL.
Flasks are filled to the brim, sealed and shipped to SLI by air freight in an insulated container to maintain ambient temperature. All shipments of cells must include media instructions.
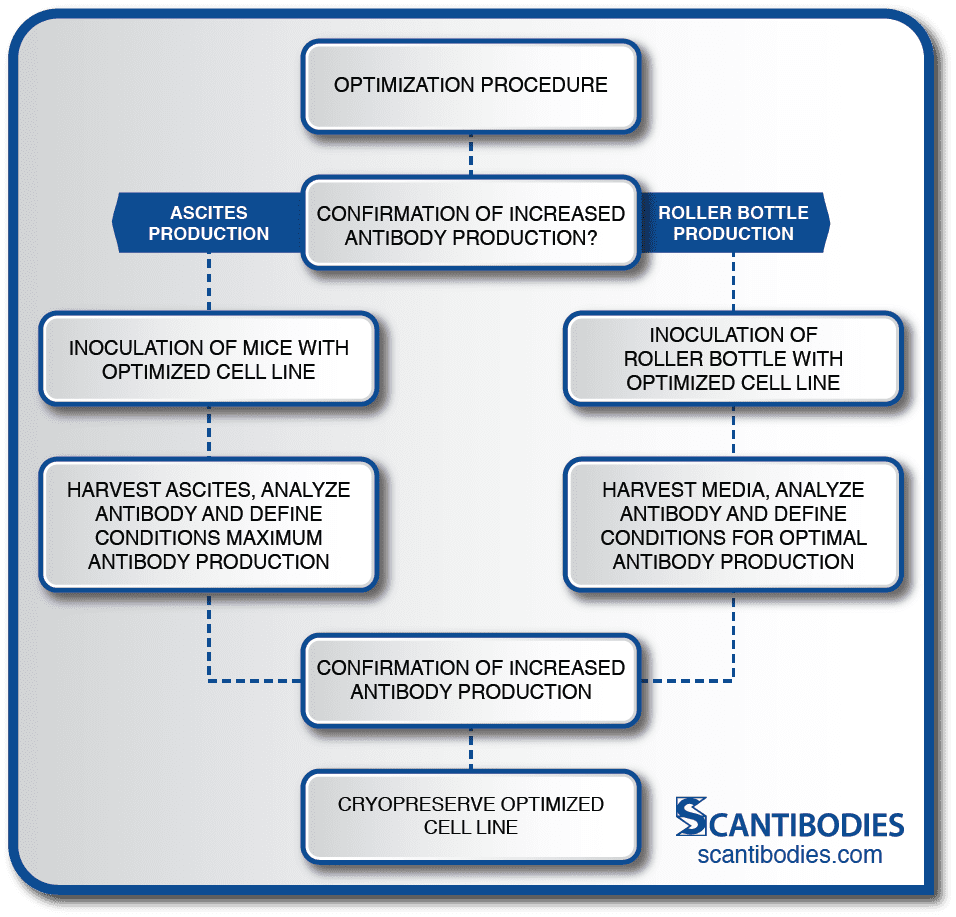
Section II: Optimization of cell line
Cell Line Optimization
The critical optimization procedure developed at SLI dramatically increases MAb production.
Small scale lots are manufactured to confirm the hybridoma’s productivity.
Roller Bottle Production
Optimal conditions for cell growth and MAb production are determined, including the appropriate media for the promotion of optimal growth, the volume of the inoculum and the appropriate operating parameters for maximum MAb production.
Roller bottles are inoculated with the selected cell line. The media is harvested according to established procedures.
Ascites Production
Optimal conditions for maximum antibody production are determined, including the pristane dose, the time interval between pristane priming and inoculation, the density of inoculum, and the age, strain and sex of mice appropriate for the MAb production.
Mice are then inoculated with 106-107 cells/mL. Ascites is harvested 2-3 weeks following inoculation. The ascitic fluid contains 10-50 times the antibody concentration of the supernatant of hybridoma cells grown in culture.
Cell lines are selected for maximum antibody production, activity and specificity using HPLC and immunoassay techniques.
To ensure the security of the selected cell line, aliquots are cryopreserved in vials located at two SLI storage sites.
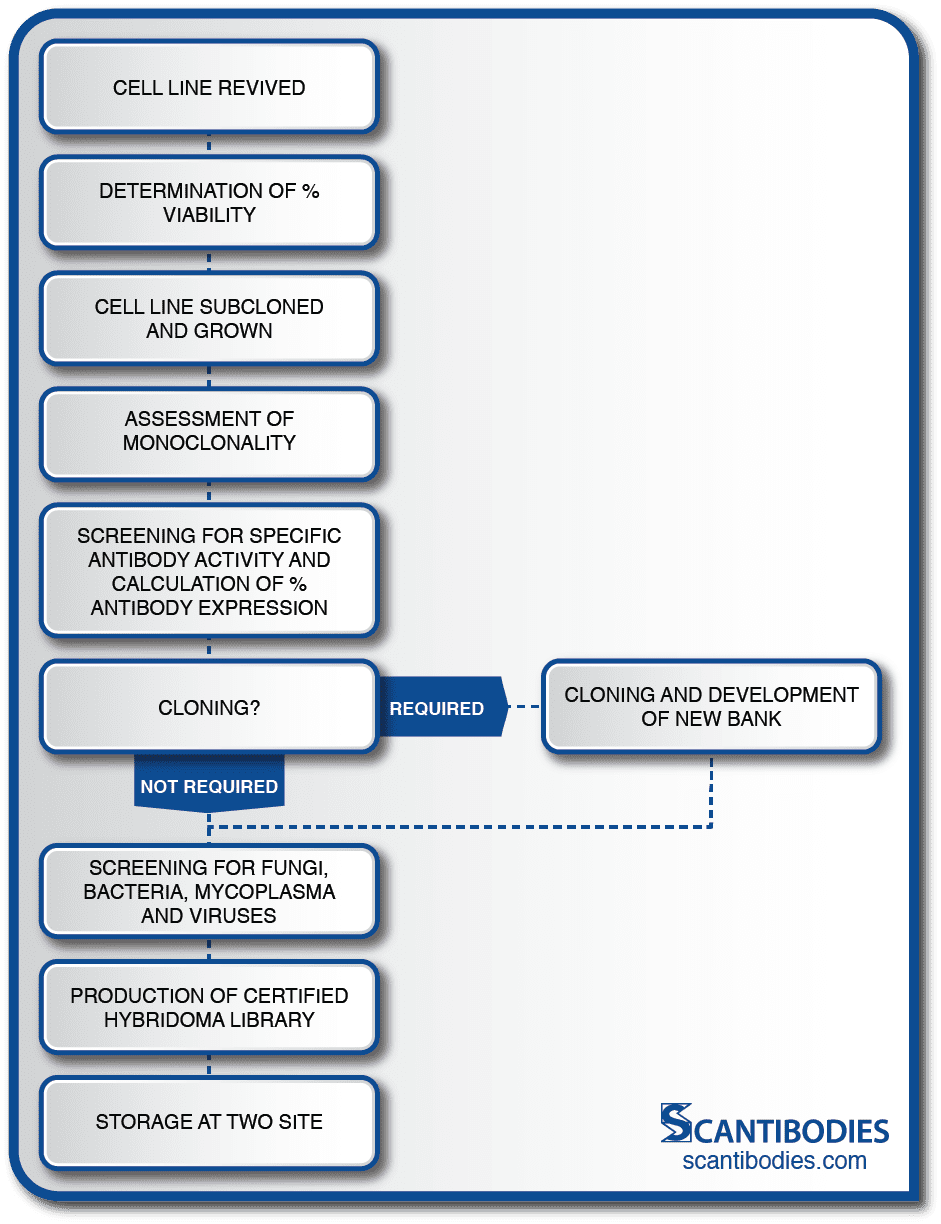
Section III: Certification of cell line
Prevention of Cell Line Drift
A cell line is comprised of a large population of cells. Over time, mutations occur within the population. Some mutations give rise to non-antibody producing cells which threaten the security of the cell line if they are permitted to grow. This condition, which is representative of one of the greatest dangers encountered by a hybridoma cell line, is referred to as cell line drift. Continuous monitoring and cell line certification is therefore essential.
At SLI, large seed stocks can be maintained for each hybridoma cell line where they are closely monitored for hybridoma viability, the absence of microbes, antibody expression and isotype uniformity.
After the cell line is revived, percent viability is determined with a trypan blue contrast assay.
The cell line is then subcloned in order to assess monoclonality. Antibody activity is assessed with an immunoassay and the percent of specific antibody expression is calculated. If necessary, the cell line is subcloned with the limiting dilution method to reinstate monoclonality preceding the development of a new cell bank.
All cell lines are screened for fungi, mold, microbial growth, mycoplasma and a panel of murine pathogens prior to the development of a certified hybridoma bank. Aliquots are stored in multiple SLI sites to ensure the security of the cell line.
Certification
Section IV: Contract In Vitro (Roller Bottle) MAb Production
Due to the absence of endogenous mouse IgG and certain serum contaminants encountered during in vivo MbB production, the purification process is simplified for roller bottle-produced MAb.
Immunological functionality for roller bottle produced MAb can be up to 10% greater than the immunological functionality for ascites produced MAb.
Roller bottles are inoculated with selected cells (generally ~107 cells per roller bottle).
Operation of the roller bottles are closely monitored to ensure adherence to defined production parameters. Media containing additives required for optimal cell growth is used. Cell growth and antibody production are assessed during culture. The harvested supernatant media is analyzed for antibody by physico-chemical analysis.
All points of the SLI Quality System are strictly adhered to for in vitro MAb production procedures.
Following the definition of MAb requirements and release specifications, a design for production is developed. Process specifications including detailed procedures, technical requirements of production facilities and equipment as well as requisites for the production staff are determined. All members of the production staff are rigorously trained, certified, periodically tested and appropriately retrained in accordance with ISO and GMP requirements.
Specifications for raw materials are defined. All raw materials used in the roller bottle production must undergo qualification procedures. Vendors from which materials are being obtained are qualified in compliance with SLI Quality System Standards. Upon arrival at SLI, all raw materials are quarantined until they are tested against set specifications.
Section V: Contract In Vivo (Mouse Ascites) MAb Production
The breeding colony at SLI maintains up to ~225,000 BALB/c mice. The colony is maintained under closely controlled conditions by animal care experts who take great interest in the welfare of the animals as well as MAb production procedures.
A breeding stock and a working stock of mice are maintained. Mice are transferred to the working stock once they have reached the maturity level required for ascites production (approximately 6 weeks).
The ventilation systems in the mouse facilities guarantees complete fresh air replacement over 15 times an hour. This maintains constant temperature and helps prevent viral or other microbial contamination.
Analogous with in vitro production methods, all points of the SLI Quality System are strictly adhered to for in vivo MAb production procedures.
The first step for in vivo MAb production is to revive the cell line.
The mice are then pristane primed and aged to promote tumor formation and enhance antibody production.
The mice are inoculated with the hybridoma cells.
Approximately 2-3 weeks following inoculation, the ascites is harvested, prepared and qualified by physico-chemical analysis for main lot pooling.
In order to prevent immunological rejection of the hybridoma by the inoculated mouse, only syngenetic animals are used for ascites production. A syngenetic animal is genetically identical, at the major histocompatibility loci, to the animal from which the hybridoma was derived.
Section VI: SLI Offers Multiple Purification Techniques for Monoclonal Antibodies
Ion Exchange Chromatography (DEAE)
DEAE separates proteins on the basis of net charge. The DEAE column support contains ions which can be competed out (exchanged) with ionic solutes in the mobile phase. This method offers a high resolving power and is capable of separating a variety of immunoglobulin types (IgG, IgM, IgD, IgA).
Protein A/G Purification
With the protein A/G method, a mixed subclass IgG preparation can be separated out by eluting a protein A or G column in a single step. This method is highly specific and can be used with almost all isotypes of IgG’s.
Alternative and/or Supplemental Purification Methods are Also Available
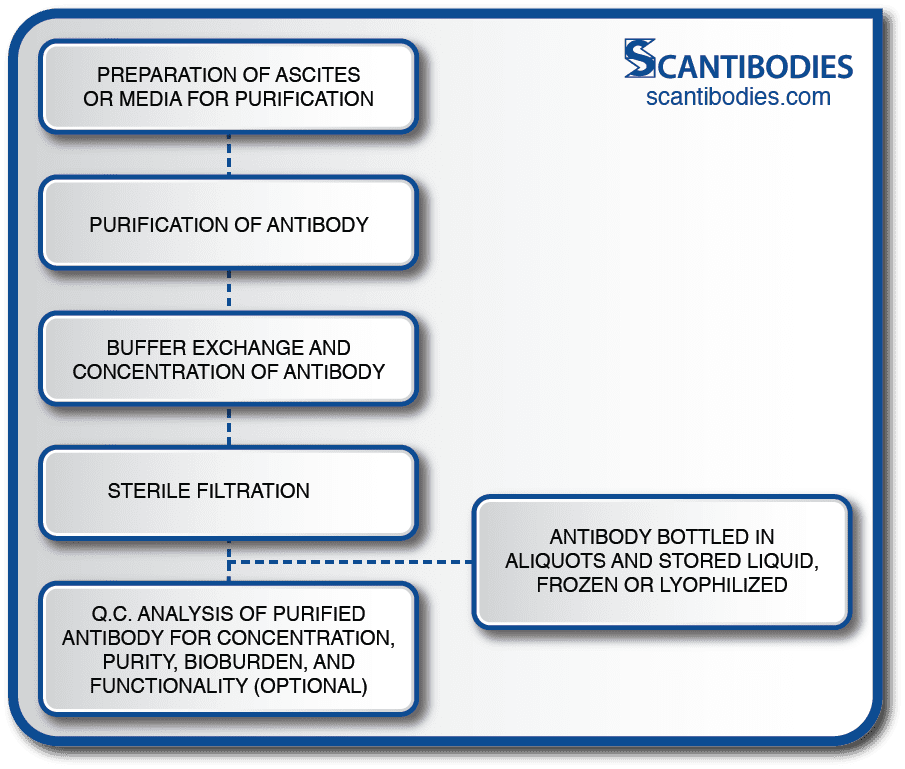
Following the purification process, the ascites or media undergoes buffer exchange, antibody concentration and aseptic filtration through a 0.2 micron filter.
The purified antibody is then bottled and stored in aliquots in liquid, frozen or lyophilized form depending on the product specifications. A Q.C. analysis assessing the purified antibody concentration, purity, bioburden and functionality (if specified) is performed.
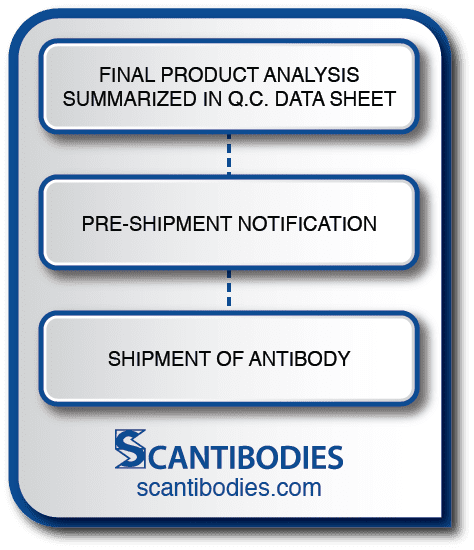
Section VII: Documentation and Shipment
Fill out our Custom Antibody Production form or call us at (619) 258-9300 for a quotation. One of our representatives will contact you.


