HETEROPHILIC BLOCKING REAGENT (HBR)
A Qualification Test for the Application of HBR
– Do you develop sandwich immunoassays?
– Have you ever tested any samples which have caused a false positive result?
– How is it possible to confirm that a sample is a false positive sample and not a true positive sample?
– Is your assay subject to HAMA or heterophilic interference?
– Have you identified the category of the false positive samples that affects your assay?
– Does your assay require a blocking reagent? If so, should the blocking reagent be a HAMA blocking reagent or a heterophilic blocking reagent?
– Do you require a universal blocking reagent which can be added to all assays
– What criteria have you established to evaluate a heterophilic blocking reagent?
Heterophilic Antibody Interference – What is It?
Heterophilic antibodies are endogenous antibodies found in patients’ serum/plasma which can bind to immunoglobulins of other species, including the species used to generate the antibodies used as reagents for immunoassays. These antibodies can interfere in immunoassay, causing a spurious elevation of measured value that is independent of the true analyte concentration, thus potentially misclassifying samples. For more details, please see our Guide to State-of-the-Art Blocking of False Positives.
Although they can affect various assay formats, their main effect is on 2-site immunometric assays.
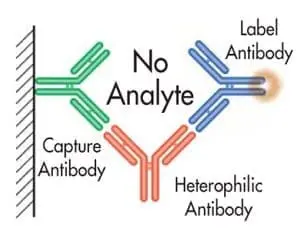
These “sandwich” assays use at least two antibodies directed against different epitopes of an antigen; one antibody is bound (or becomes bound) to a solid-phase, while the other is in solution and tagged with a signal moiety such as 125-I, enzyme, fluorophore, CLIA label, etc. Normally, antigen present in the sample “bridges” the two antibodies so that the amount of labeled antibody which becomes bound to the solid-phase is proportional to the antigen concentration in the sample.
Figure 1

However, heterophilic antibodies can also “bridge” the two antibodies independently of antigen, resulting in an increase in bound labeled antibody concentration.
Figure 2
The Unique Aspects of HBR over Conventional Blocking Methods
Conventional, passive blocking methods use nonspecific substances (mouse IgG, mouse serum, nonspecific monoclonal antibodies, aggregated IgG, etc.) to block the binding of the human heterophilic antibody. All of these approaches rely on the affinity of the human heterophilic antibody to affect the blocking. The affinity of the human heterophilic antibody is typically in the K-value range of 105-106.
HBR accomplishes its binding by a totally different approach. The HBR is a specific binder that is directed against the human heterophilic antibody. When HBR binds to the human heterophilic antibody, the blocking is accomplished by steric hindrance. The HBR blocking is effected by the specific binder which has an affinity in the range of K = 109
The specific binding action of the HBR, coupled with the thousand times higher affinity in the reaction, results in the following advantages of HBR over conventional blocking methods:
– With HBR, less protein is required for blocking (no decrease in assay signal).
– HBR blocks more false positives than are blocked with conventional methods.
– HBR blocks all anti-species (anti-rabbit, anti-goat, anti-sheep, as well as anti-mouse).
– HBR blocks all anti-subtypes of mouse monoclonals (example: anti-mouse IgG1, anti-mouse IgG2, etc.); whereas, the use of one monoclonal antibody such as IgG1, will only block human antibodies to that subspecies of monoclonal antibodies.
– The use of HBR does not have to be avoided in certain tests, as in the case in which an analyte-specific monoclonal antibody is used for blocking.
Heterophilic Interference – What is Known?
– The interfering factor is an immunoglobulin, and both IgG and IgM heterophilic antibodies have been reported.
– They occur at a high incidence. Depending upon patient population, up to 40% incidence has been reported, and the existence of at least 10% incidence has been documented.
– The magnitude of the interference varies from sample to sample, and may vary within a patient over time.
– The heterophilic antibodies are not species specific, but can bind to a variety of animal antibodies. Thus, the interference is not limited to monoclonal antibody-based assays.
– The interference is probably mediated via the Fc region of the antibodies used in the assay.
– Heterophilic antibodies are not a single, specific entity, but are a multi-component phenomenon. They comprise a mixed population of antibodies which can cause interference, some or all of which may be present in a particular sample.
– EIA’s appear to be more susceptible than RIA’s, possibly because of the increased modification of the Fc region during conjugation.
Heterophilic Antibodies – Sources
There is a variety of possible causes for inducing heterophilic antibodies in patients, including:
– Exposure to animals (e.g. animal technicians, veterinarians, animal handlers)
– Alternate animal contact therapy (e.g. thymic cells, sheep cells, embryonic cells)
– Exposure to animal products (e.g. food preparation)
– Special diets (e.g. cheese)
– Deliberate immunization (e.g. therapies, vaccinations, certain imaging treatments)
– Rheumatoid factors can also act as heterophilic antibodies
– Blood transfusions
– Autoimmune diseases
– Dialysis
– Patent medicines (OKT3)
– Maternal transfer
– Cardiac Myopathy
– G.I. Disease (E. Coli)
Heterophilic Interference – How is it Detected?
A variety of methods have been proposed:
– Discordant values from the clinical picture or another reference assay.
– Poor dilution performance of certain samples in an assay with normally satisfactory performance.
– Removal of specific analyte from sample by affinity chromatography to see if signal is abolished (if not, then it is a false positive).
– Addition of heterophilic blocking reagent to see if observed value is decreased.
What is the Difference Between “Heterophilic Antibody” and “HAMA”?
“Heterophilic antibody” is a generic term used to describe all human antibodies which can bind to animal antibodies and cause interference in immunoassays.
“HAMA” (human anti-mouse antibody) is one type of heterophilic antibody which can bind to mouse antibodies.
What is a Blocking Reagent?
A Blocking Reagent is a preparation which, when added to immunoassay reagents, prevents non-analyte mediated bridging of antibodies by heterophilic interference.
There are two main types of blocking reagent:
“HAMA” type
These block only one specific human anti-species antibody activity (e.g. human anti-mouse), and are typically normal serum, normal IgG, monoclonal antibody not directed against the target analyte, etc.
They are “passive blocking agents” in that they are added in excess concentration so that any specific anti-species antibodies present in the sample bind to these in preference to the specific immunoreactants present in low concentrations.
Such reagents are of limited use as they only remove one component of the heterophilic interference, which is a multi-component phenomenon. It is frequently observed that addition of mouse IgG (for example) to a double-monoclonal sandwich assay will only correct a portion of the heterophilic interference.
True Heterophilic Blocking Reagents
These are formulations which have the ability to remove all types of heterophilic interference. Some are “active blocking reagents” in that they are directed specifically against the interfering heterophilic antibodies, allowing them to be used at lower concentrations, (thus minimizing any adverse effects on the immunoassay reaction), and with enhanced effects on blocking kinetics.
Characteristics of an Ideal Heterophilic Blocking Reagent
– It should have the ability to correct interference from all samples, irrespective of whether it is caused by specific anti-species activity, rheumatoid factor, etc.
– It should be effective at low concentrations so as not to interfere with the dose-response curve.
– It should not interfere with spike and recovery of the analyte in human serum.
– It should not interfere with linearity of dilution of a true positive patient sample.
– It should exhibit reproducible performance with no lot-to-lot variation.
– It should have long term stability.
– Its cost should be such that incorporation is economically viable.
– It should be suitable for incorporation into one of the immunoreagents to obviate the requirement for an additional reagent or a separate sample pretreatment step.
The Scantibodies Heterophilic Blocking Reagent (HBR)
Scantibodies HBR is a novel reagent which has been specifically designed to combat the problems of heterophilic antibody interference in immunoassays. It is a unique formulation of immunoglobulins targeted specifically against heterophilic antibodies to neutralize their interference. HBR is a defined reagent with a purity >95%.
Unlike most of the non-specific “passive blockers” which are available from other suppliers (which need to be added in vast excess to ensure that heterophilic antibodies will bind to them in preference to assay-specific components), Scantibodies’ HBR is an “active blocker”. This formulation of immunoglobulins is targeted specifically against heterophilic antibodies and is therefore able to neutralize their interference.
Performance Characteristics
1. HBR blocks false positive reactions from a panel of:
– human anti-mouse antibodies (HAMA)
– human anti-goat antibodies
– human anti-sheep antibodies
– human anti-rabbit antibodies
– rheumatoid factor
2. The broad specificity means that Scantibodies HBR is suitable as a “Universal Reagent” for 2-site immunometric assays using any of the commonly employed antibodies as solid-phase or labeled reagents.
3. Scantibodies HBR, being a specific, highly purified reagent, can be used at very low concentrations (sometimes 100x’s or 1,000x’s less than normal mouse IgG) so that the assay signal is not adversely affected.
4. Scantibodies HBR does not interfere with spike and recovery of analyte in human serum.
5. Scantibodies HBR does not interfere with linearity of dilution of a true positive patient sample.
6. Scantibodies HBR is highly reproducible from lot to lot.
7. Scantibodies HBR is stable for up to 5 years.
8. The price of incorporation of Scantibodies HBR in an assay is less than the cost of using mouse IgG or mouse serum.
9. Scantibodies HBR can be added directly to assay buffers, conjugates, etc
False Positive Identification
Scantibodies has identified donors which elicited false positive results. These donors were the most difficult to block with conventional methods (mouse IgG, etc.) These samples may be used to help identify false positives and evaluate the vulnerability of an assay format against heterophilic interaction. Please note that false positive samples are very often assay specific. Therefore, these samples may not show false positives in certain assays.
The target samples of heterophilic specimens are valuable to have in developing a reagent blocking formulation. These samples are typically assay dependent and are best obtained through a large sample screening using the actual assay for which the reagent blocking formulation is being developed. Scantibodies offers this custom screening program of up to 10,000 samples with a potential to make available 225 ml from each donor sample.
Certification
Scantibodies’ HBR is supplied with a data sheet detailing the following:
1. Purity by SDS-PAGE
2. Buffer composition
3. Storage conditions
4. Stability
5. Appearance
6. No preservatives
HBR is supplied in liquid form.
For pricing details or to arrange for a FREE sample of HBR, please contact Scantibodies Laboratory.
Blocking Reagents for Assay Manufacturers
It is well documented that heterophilic antibodies are one of the major causes responsible for falsely elevated results. However, other types of heterophilic interactions are far from fully understood. The heterophilic interaction also is antibody and assay specific: The same heterophilic serum may act differently on difference antibody pair or assay formats. In addition, non-specific interaction also contributes to the falsely elevated results. Therefore, SLI developed different HBR formulations in order to have a wide variety of selections to reach a fine balance in addressing the different types of interactions and assay applications. Scantibodies has developed superior quality passive and active blockers.
PASSIVE BLOCKERS
Mouse Immunoglobulin (IgG)
Mouse IgG acts as a passive heterophilic blocker that neutralizes a subpopulation of the heterophilic antibody and reduces the heterophilic interactions. In addition, Mouse IgG also increases the free IgG concentration in an assay system and reduces the non-specific interaction that results in falsely elevated analyte concentrations. It is an inexpensive blocker that works well in a good number of assay formats. However, it does not have the active blocking ability as HBR does. Therefore, Mouse IgG may not be powerful enough to completely block certain strong heterophilic interactions.
Mouse IgG, Purified (Part# 3BM245)
This product is affinity-purified from normal mouse serum at a concentration of 10-12 mg/ml. It contains all the normal subclasses of mouse IgG, and is provided at a purity of greater than or equal to 95%. Mouse IgG has known characteristics for passive blocking efficacy which makes it a good choice for a heterophilic blockage at a lower cost. Therefore, it is recommended for heterophilic blockage in immunoassays in which both the capture and detection antibodies are of murine origin.
Mouse IgG, Purified, Concentrated (Part# 3BM845)
This is a more concentrated (>20 mg/ml) version of our Mouse IgG.
Mouse IgG, Purified (Part# 3BM203)
It has a concentration of 10-12mg/ml, Buffer: 0.1M Sodium Phosphate buffer, pH 7.4, 0.1% NaN3.
————
Goat IgG (Immunoglobulin G), Liquid (Part# 3BG247)
It has a concentration of 10-30mg/ml, Buffer: 0.01M PBS, pH 7.4, 0.1% NaN3.
Goat IgG (Immunoglobulin G), Lyophilized (Part# 3BG568)
It has a concentration of 45-60mg/ml, Buffer: PBS(3mM Potassium Phosphate, 10mM Sodium Phosphate, 115mM Sodium Chloride, pH 7.2)no preservative added.
————
Rabbit IgG (Part# 3BR230)
It has a concentration of 5-15mg/ml, Buffer: 0.01M PBS, pH 7.4, no preservative added.
————
Sheep IgG (Part# 3BS251)
It has a concentration of 4.5 -5.5 mg/ml, Buffer 0.01M Phosphate buffer, 0.117M Sodium Chloride, pH 7.2, no preservative added.
ACTIVE BLOCKERS
Assay Development Blocking Kit (Part# 3KG775)
This kit has been designed for those products that are currently in assay development. It contains 10 of our different HBR products (including our HBR 20 series) all of which have been carefully selected to be used as an aid in developing a reagent blocking formulation to eliminate false positive or negative interferences, and to ensure that they are useful for both active and passive blocking.
HBR-1- Purified (Part# 3KC533, 20 mg/ml)
It contains specific murine immunoglobulins that block the heterophilic interaction by active binding to the heterophilic antibodies, which are capable of cross linking the capture and the detection antibodies used in the immunoassay, resulting in false positive readings. The attachment of HBR-1 to the heterophilic antibodies blocks this cross-linking, and eliminates the interference caused by the heterophilic antibodies in the humoral fluids. In addition to its active blocking, this product is also characteristic of its passive blockage of the heterophilic interaction as well. The HBR is a liquid reagent with a protein concentration of 20 ± 2 mg/ml. The immunoglobulins are dissolved in a phosphate buffer with a pH of 7.4. The immunoglobulins in this product are at a purity of greater than or equal to 95% as shown by SDS-PAGE.
HBR-3, Purified (Part# 3KC576)
Each vial contains approximately 4 mg of murine immunoglobulins. This product represents a variation in formulation with similar essential characteristics compared to HBR-1-Purified. The special formulation is designed to enhance its blocking capability at a lower concentration of immunoglobulins.
HBR-6, Purified (Part# 3KC542)
This HBR-6 is specially formulated to enhance its heterophilic blocking ability. The immunoglobulins in this reagent are at a purity of greater than or equal to 95%. It has a concentration of 3-6 mg/ml.
HBR-9, Purified (Part# 3KC564)
The HBR-9 is also one of our newly formulated products developed as an alternative for the HBR Plus, 3KC545. It contains murine immunoglobulins with different characteristics. It is specially formulated for application for immunoassays in which both the capture and detection antibodies are of murine origin. Like HBR Plus, this product is characteristic for its active as well as passive blocking efficacy. Each vial of this product contains approximately 20 mg of immunoglobulins, which are at a purity of greater than 90%, as shown by SDS-PAGE.
HBR-11, Purified (Part# 3KC565)
3KC565 has been formulated with murine immunoglobulins. In addition to the products listed above, HBR-11 provides our customers with more selection for heterophilic blockage. The immunoglobulins in this product are at a purity of greater than or equal to 90%. It has a concentration of 10.0 +/- 1.0 mg/ml.
HBR Plus, Purified (Part# 3KC545)
3KC545 was developed as an alternative for HBR-1. This product is compounded with immunoglobulins with different characteristics. Therefore, in addition to its active blocking characteristics, the special formulation and production procedures enhance its efficacy in its passive blocking ability as well. It has a concentration of 10.0 +/- 1.0 mg/ml.
HBR-21 (Part# 3KC002)
3KC545 was developed as an alternative for HBR-1. This product is compounded with immunoglobulins with different characteristics. Therefore, in addition to its active blocking characteristics, the special formulation and production procedures enhance its efficacy in its passive blocking ability as well. It has a concentration of 10.0 +/- 1.0 mg/ml.
HBR-22 (Part# 3KC003)
The HBR-22 is also one of our newly formulated products. In addition to the products listed above, the HBR-22 provides our customers with more selection for heterophilic blockage. Each vial of this product contains approximately 10 mg of immunoglobulins.
HBR-23 (Part# 3KC006)
The HBR-23 is also one of our newly formulated products. This product is formulated with murine immunoglobulins. In addition to the products listed above, the HBR-23 provides our customers with more selection for heterophilic blockage. Each vial of this product contains approximately 10 mg of immunoglobulins.
HBR-24 (Part# 3KC007)
The HBR-24 is also one of our newly formulated products. This product is formulated with murine immunoglobulins. In addition to the products listed above, the HBR-24 provides our customers with more selection for heterophilic blockage. Each vial of this product contains approximately 10 mg of immunoglobulins.
Blocking Reagents for Clinical Labs, Hospitals, etc.
Heterophilic Blocking Tube (HBT) (Part# 3IX762)
3IX762 contains a unique blocking reagent composed of specific binders which inactivate heterophilic antibodies. Once the specific binders have bound to the heterophilic antibodies, the antibodies are no longer able to cause immunoassay interference. The reagent is in the form of a lyophilized pellet at the bottom of the tube and each tube contains enough reagents to inactivate the heterophilic antibodies in 500 ?l of sample material. The HBT also allows for the rapid and simple elimination of false positive heterophilic interference in plasma or serum for immunoassays (i.e., FSH, LH, Prolactin, TSH, Ferritin, CEA, AFP, hCG, HBsAg, CK-MB, CA 125, CA 19-9, NSE, etc.).
Non-Specific Antibody Blocking Tube (NABT) (Part# 3IX761)
3IX761 contains immunoglobulins which the non-specific antibodies in the serum or plasma samples bind to, thus causing them to be blocked from interfering in antibody detection immunoassays. The NABT also allows for the rapid and simple elimination of false positive non-specific antibody interference in plasma or serum for detection assays (i.e., anti-HCV, HIV, Toxoplasmosis, Rubella, CMV, Herpes, Tg, Thyroglobulin, TPO Thrombopoietin, etc.). The reagent is in the form of a lyophilized pellet at the bottom of the tube and each tube contains enough reagents to inactivate the non-specific antibodies in 500 ?l of sample material.